[ps2id id=’study-setting’ target=”/]
9. STUDY SETTING
Description of study settings (e.g., community clinic, academic hospital) and list of countries where data will be collected. Reference to where list of study sites can be obtained.
Example
“Selection of Countries
. . . To detect an intervention-related difference in HIV incidences with the desired power, the baseline incidences at the sites must be sufficiently high. We chose the participating sites so that the average baseline annual incidence across all communities in the study is likely to reach at least 3%. The various sites in sub-Saharan Africa met this criterion, but we also wanted sites in Asia to extend the generalizability of the intervention. The only location in Asia with sufficient incidence at the community level is in ethnic minority communities in Northern Thailand, where HIV incidence is currently in excess of 7%;[Reference X] thus they were invited to participate as well. Our final selection of sites combines rural (Tanzania, Zimbabwe, Thailand, and KwaZulu-Natal) and an urban (Soweto) location. The cultural circumstances between the sub-Saharan African sites vary widely . . .
Definition of Community
Each of the three southern African sites (Harare, Zimbabwe; and Soweto and Vulindlela, South Africa) selected eight communities, the East African (Tanzanian) site selected 10 communities, and Thailand selected 14 communities . . . They are of a population size of approximately 10,000 . . . which fosters social familiarity and connectedness, and they are geographically distinct. Communities are defined primarily geographically for operational purposes for the study, taking into account these dimensions of social communality. The communities chosen within each country and site are selected to be sufficiently distant from each other so that there would be little cross-contamination or little possibility that individuals from a control community would benefit from the activities in the intervention community.” 113
Explanation
A description of the environment in which a trial will be conducted provides important context in terms of the applicability of the study results; the existence and type of applicable local regulation and ethics oversight; and the type of healthcare and research infrastructure available. These considerations can vary substantially within and between countries.
At a minimum, the countries, type of setting (e.g., urban versus rural), and the likely number of study sites should be reported in the protocol. These factors have been associated with recruitment success and degree of attrition for some trials,68;91;92;114-117 but not for others.118;119 Trial location has also been associated with trial outcome,120 aspects of trial quality (e.g., authenticity of randomisation121), and generalizability[ps2id id=’eligibility-criteria’ target=”/].122
10. ELIGIBILITY CRITERIA
Inclusion and exclusion criteria for participants. If applicable, eligibility criteria for study centres and individuals who will perform the interventions (e.g., surgeons, psychotherapists).
Example 1
“Patients (or a representative) must provide written, informed consent before any study procedures occur (see Appendix 1 for sample Informed Consent Form) . . .
5.1. Inclusion Criteria
Patients eligible for the trial must comply with all of the following at randomization:
- Age ≥16 years
- Current admission under the care of the heart-failure service at the site
. . .
5.2. Exclusion Criteria
- Acute decompensation thought by the attending heart-failure physician to require or be likely to require PAC [pulmonary-artery catheter] during the next 24 hours. Such patients should be entered into the PAC Registry (see below).
- Inability to undergo PAC placement within the next 12 hours
. . .
Patients enrolled in other investigational drug studies are potential candidates for ESCAPE. As the ESCAPE protocol does not involve any investigational agents or techniques, patients would be eligible for dual randomization if they are on stable doses of the investigational drugs . . .
13. Study Network, Training, and Responsibilities
. . . To qualify, physicians responsible for PAC [pulmonary-artery catheter] placements will be required to show proof of insertion of ≥50 PACs in the previous year with a complication rate of <5%. Further, clinicians will need to show competence in the following areas to participate in the study: 1) insertion techniques and cardiovascular anatomy; 2) oxygen dynamics . . . and 7) common PAC complications.[Reference X] . . . we will assume basic competence in these areas after satisfactory completion of the PACEP [PAC educational programme] module.” 123
Example 2
“Trial centre requirements
A number of guidelines have stated thrombolysis should only be considered if the patient is admitted to a specialist centre with appropriate experience and expertise[Reference X]. Hospitals participating in IST-3 [third International Stroke Trial] should have an organized acute stroke service. The components of effective stroke unit care have been identified . . . In brief, the facilities (details of these requirements are specified in the separate operations manual) should include:
- Written protocol for the acute assessment of patients with suspected acute stroke to include interventions to reduce time from onset to treatment.
- Immediate access to CT [Computerised Tomographic] or MR [Magnetic Resonance] brain scanning (preferably 24 hours a day).
- A treatment area where thrombolysis may be administered and the patient monitored according to trial protocol, preferably an acute stroke unit.” 124
Explanation
Eligibility criteria for potential trial participants define the study population. They can relate to demographic information; type or severity of the health condition; comorbidities; previous or current treatment; diagnostic procedures; pregnancy; or other relevant considerations.125 In trials of operator-dependent interventions such as surgery and psychotherapy, it is usually important to promote consistency of intervention delivery by also defining the eligibility criteria for care providers and centres where the intervention will be administered.126
Clear delineation of eligibility criteria serves several purposes. It enables study personnel to apply these criteria consistently throughout the trial.127 The choice of eligibility criteria can affect recruitment and attrition,67;114;115;117;118;128-130 as well as outcome event rates.39;131 In addition, the criteria convey key information related to external validity (generalisability or applicability).132 The importance of transparent documentation is highlighted by evidence that the eligibility criteria listed in publications are often different from those specified in the protocol.125;133;134
Certain eligibility criteria warrant explicit justification in the protocol, particularly when they limit the trial sample to a narrow subset of the population.132;135;136 The appropriateness of restrictive participant selection depends on the trial objectives.137 When trial participants differ substantially from the overall population to whom the intervention will be applied, the trial results may not reflect the impact in real world practice settings[ps2id id=’interventions’ target=”/].134;138-144
11. INTERVENTIONS
11a. Interventions
Interventions for each group with sufficient detail to allow replication, including how and when they will be administered.
Example
“Eligible patients will be randomised in equal proportions between IL-1ra [interleukin-1 receptor antagonist] and placebo, receiving either a once daily, subcutaneous (s.c.) injection of IL-1ra (dose 100 mg per 24 h) for 14 days, or a daily s.c. injection of placebo for 14 days . . .
The study drug and placebo will be provided by Amgen Inc in its commercially available recombinant form . . . The study drug and placebo will be relabelled by Amgen, in collaboration with CTEU [Clinical Trials and Evaluation Unit] according to MHRA [Medicines and Healthcare Regulatory Agency] guidelines.
The first dose of IL-1ra will be given within 24 h +2 h of the positive Troponin. Injections will be given at a standardised time (24 ± 2 h after the previous dose), immediately after blood sampling. IL-1ra or placebo will [be] administered to the patient by the research nurse while the patient is in hospital. During the hospital stay, the patient will be taught to self-administer the injection by the research nurse and on discharge will continue at home. This has proven possible in other ACS [acute coronary syndrome] trials that required self injection of subcutaneous heparin [Reference X]. Full written guidance on self injection will also be provided to patients. If self injection is found not to be possible in an individual patient for unexpected reasons, an alternative method will be sought (e.g. district nurse, or attending the hospital) to try and maintain full compliance with scheduled study drug regimen after discharge. Patients will also be asked to complete a daily injection diary. All personnel will be blinded to the identity of the syringe contents.” 145
Explanation
Studies of trials and systematic reviews have shown that important elements of the interventions are not described in half of the publications.146;147 If such elements are also missing from the protocol, or if the protocol simply refers to other documents that are not freely accessible, then it can be impossible for healthcare providers, systematic reviewers, policymakers, and others to fully understand, implement, or evaluate the trial intervention.148 This principle applies to all types of interventions, but is particularly true for complex interventions (e.g., health service delivery; psychotherapy), which consist of interconnected components that can vary between healthcare providers and settings.
For drugs, biological agents, or placebos, the protocol description should include the generic name, manufacturer, constituent components, route of administration, and dosing schedule (including titration and run-in periods, if applicable).149;150 The description of non-drug interventions – such as devices, procedures, policies, models of care, or counselling – is generally more complex and warrants additional details about the setting (Item 9) and individuals administering the interventions. For example, the level of pre-trial expertise (Item 10) and specific training of individuals administering these complex interventions are often relevant to describe (e.g., for surgeons, psychologists, physiotherapists). When intervention delivery is subject to variation, it is important to state whether the same individuals will deliver the trial interventions in all study groups, or whether different individuals will manage each study group – in which case it can be difficult to separate the effect of the intervention from that of the individual delivering it. Interventions that consist of ‘usual care’ or ‘standard of care’ require further elaboration in the protocol, as this care can vary substantially across centres and patients, as well as over the duration of the trial.[ps2id id=’modifications’ target=”/]
See TIDieR checklist for further recommendations on intervention descriptions
11b. Modifications
Criteria for discontinuing or modifying allocated interventions for a given trial participant (e.g., drug dose change in response to harms, participant request, or improving/worsening disease).
Example
“Gastro-Intestinal Upset
The tablets may be taken in two equally divided doses, if necessary, to improve gastro-intestinal tolerance. Should it be necessary the daily dose may be reduced by one tablet at a time to improve gastro-intestinal tolerance.
Renal Function Impairment
Since sodium clodronate is excreted unchanged by the kidney its use is contra-indicated in patients with moderate to severe renal impairment (serum creatinine greater than 2 times upper limit of normal range of the centre). If renal function deteriorates to this extent the trial medication should be withdrawn from the patient. This should be reported as an adverse event. In patients with normal renal function or mild renal impairment (serum creatinine less than 2 times upper limit of normal range of the centre) serum creatinine should be monitored during therapy.
Allergic Reactions
Allergic skin reactions have been observed in rare cases. If this is suspected withdraw the trial medication from the patient. This should be reported as an adverse event.
Biochemical Disturbances
Asymptomatic hypocalcaemia has been noted rarely. Temporary suspension of the trial medication until the serum calcium returns into the normal range is recommended. The trial medication can be then restarted at half the previous dose. If the situation returns withdraw the trial medication from the patient. This should be reported as an adverse event . . .” 151
Explanation
For a given trial participant, the assigned study intervention may need to be modified or discontinued by trial investigators for various reasons, including harms, improved health status, lack of efficacy, and withdrawal of participant consent. Comparability across study groups can be improved, and subjectivity in care decisions reduced, by defining standard criteria for intervention modifications and discontinuations in the protocol. Regardless of any decision to modify or discontinue their assigned intervention, study participants should be retained in the trial whenever possible to enable follow-up data collection and prevent missing data (Item 18b)[ps2id id=’adherence’ target=”/].152
11c. Adherence
Strategies to improve adherence to intervention protocols, and any procedures for monitoring adherence (e.g., drug tablet return; laboratory tests).
Example
“Adherence Reminder Sessions
Face-to-face adherence reminder sessions will take place at the initial product dispensing and each study visit thereafter. This session will include:
- The importance of following study guidelines for adherence to once daily study product
- Instructions about taking study pills including dose timing, storage, and importance of taking pills whole, and what to do in the event of a missed dose.
- Instructions about the purpose, use, and care of the MEMS® cap [medication event monitoring system] and bottle
- Notification that there will be a pill count at every study visit
- Reinforcement that study pills may be TDF [tenofovir disproxil fumarate] or placebo
- Importance of calling the clinic if experiencing problems possibly related to study product such as symptoms, lost pills or MEMS® cap.
Subsequent sessions will occur at the follow-up visits. Participants will be asked about any problems they are having taking their study pills or using the MEMS® cap. There will be brief discussion of reasons for missed doses and simple strategies for enhancing adherence, e.g., linking pill taking to meals or other daily activities. Participants will have an opportunity to ask questions and key messages from the initial session will be reviewed as needed . . .
Adherence assessments
To enhance validity of data, multiple methods will be used to assess medication adherence including pill count; an electronic medication event monitoring system (MEMS® cap) [Reference X]; and ACASI [audio-computer administered interview] questionnaire items including a one month visual analogue scale [Reference X], reasons for non-compliance, and use of the MEMS® cap. Participants will return the unused tablets and bottle at each follow-up visit. Unused tablets will be counted and recorded on the appropriate CRF [case report form]. Electronic data collected in the MEMS® cap will be downloaded into a designated, secure study computer.” 153
Explanation
Adherence to intervention protocols refers to the degree to which the behaviour of trial participants corresponds to the intervention assigned to them.154 Distinct but related concepts include trial retention (Item 18b) and adherence to the follow-up protocol of procedures and assessments (Item 13).
On average, adherence to intervention protocols is higher in clinical trials than in non-research settings.155 Although there is no consensus on the acceptable minimum adherence level in clinical trials, low adherence can have a substantial impact on statistical power and interpretation of trial results.156-158 Since fewer participants are receiving the full intervention as intended, non-adherence can reduce the contrast between study groups – leading to decreased study power and increased costs associated with recruiting larger sample sizes for evaluating superiority, or leading to potentially inappropriate conclusions of non-inferiority or equivalence. There is also the possibility of underestimating any efficacy and harms of the study intervention.
Furthermore, if adherence is a marker for general healthy behaviour associated with better prognosis, then different rates of non-adherence between study groups can lead to a biased estimate of an intervention’s effect. In support of this ‘healthy adherer’ effect, non-adherers to placebo in clinical studies have been found to have poorer clinical outcomes than adherers.159
To help avoid these potential detrimental effects of non-adherence, many trials implement procedures and strategies for monitoring and improving adherence,67;156-158 and any such plans should be described in the protocol.160 Among applicable drug trials published in 1997-99, 47% reported monitoring the level of adherence.161 Although each of the many types of monitoring methods has its limitations,157;158 adherence data can help to inform the statistical analysis (Item 20c), trial interpretation, and choice of appropriate adherence strategies to implement in the trial as it progresses or in future trials and clinical practice.
A variety of adherence strategies exist,156-158 and their use can be tailored to the specific type of trial design, intervention, and participant population. It may be desirable to select strategies that can be easily implemented in clinical practice, so that the level of adherence in the real-world setting is comparable to that observed in the trial[ps2id id=’concomitant-care’ target=”/].158
11d. Concomitant care
Relevant concomitant care and interventions that are permitted or prohibited during the trial.
Example
“2. Rescue Medication
For weeks 0-3, topical mometasone furoate 0.1% cream or ointment (30 g/week) will be permitted with participants preferably using ointment. Participants will be instructed to apply the topical mometasone furoate to blisters/lesions as required (not to areas of unaffected skin). If the participant is allergic to mometasone furoate or the hospital pharmacy does not stock it, then an alternative topical steroid may be prescribed but this must be in the potent class. In addition, participants will be advised that they can apply a light moisturiser to blisters/lesions at any time during the study.
For weeks 3-6, use of mometasone furoate (or other topical corticosteroids) is strongly discouraged to prevent potential systemic effects. Accidental use of mometasone furoate or other potent topical steroid during this period will be classified as a protocol deviation.
After week 6, potent topical corticosteroids (up to 30 g/week) may be used to treat symptoms and localised disease if they would have normally been used as part of normal clinical care by the physician in charge of that patient. This must be recorded on the trial treatment log.
However, those patients who are on a dose reducing regime for oral steroids, 30 g/week of a ‘potent’ topical steroid will be allowed.
3. Prohibited Concomitant Medications
The administration of live virus vaccines is not permitted for all participants during weeks 0-6 as the investigator is blinded to treatment allocation, and must therefore warn all participants to refrain for [sic] having a live virus vaccine. However, after week 6, once the investigator knows which medication the participant is on, only those taking prednisolone will not be allowed live virus vaccines.
Participants should continue to take medications for other conditions as normal. However, if it is anticipated that the participant will need a live virus vaccine during the intervention phase, they will be ineligible for entry into the study.” 50
Explanation
In a controlled trial, a key goal is to have comparable study groups that differ only by the intervention being evaluated, so that any difference in outcomes can be attributed to effects of the study intervention. Co-intervention bias can arise when the study groups receive different concomitant care or interventions (in addition to the assigned trial interventions) that may impact trial outcomes.162 To promote comparability of study groups, the protocol should list the relevant concomitant care and interventions that are allowed (including rescue interventions), as well as any that are prohibited.[ps2id id=’outcomes’ target=”/]
12. OUTCOMES
Primary, secondary, and other outcomes, including the specific measurement variable (e.g., systolic blood pressure), analysis metric (e.g., change from baseline, final value, time to event), method of aggregation (e.g., median, proportion), and time point for each outcome. Explanation of the clinical relevance of chosen efficacy and harm outcomes is strongly recommended.
Example
“1. Primary Outcome Measures
• Difference between the two treatment arms in the proportion of participants classed as treatment success at 6 weeks. Treatment success is defined as 3 or less significant blisters present on examination at 6 weeks. Significant blisters are defined as intact blisters containing fluid which are at least 5 mm in diameter. However, if the participant has popped a blister, or the blister is at a site that makes it susceptible to bursting such as the sole of the foot, it can be considered part of the blister count, providing there is a flexible (but not dry) roof present over a moist base. Mucosal blisters will be excluded from the count.
A survey of the UK DCTN [United Kingdom Dermatology Clinical Trials Network] membership showed that a point estimate of 25% inferiority in effectiveness would be acceptable assuming a gain in the safety profile of at least 10%.
This measure of success was selected as it was considered to be more clinically relevant than a continuous measure of blister count. It would be less clinically relevant to perform an absolute blister count and report a percentage reduction. Instead, to state that treatment is considered a success if remission is achieved (i.e. the presence of three or less blisters on physical examination at 6 weeks) more closely reflects clinical practice. In addition, it is far less burdensome on investigators than including a full blister count, which would mean counting in the region of 50 – 60 blisters in many cases. This outcome measure will be performed as a single blind assessment.
• Difference between the two treatment arms in the proportion of participants reporting grade 3, 4 and 5 (mortality) adverse events which are possibly, probably or definitely related to BP [Bullous Pemphigoid] medication in the 52 weeks following randomisation. A modified version of The Common Terminology Criteria for Adverse Events (CTCAE v3.0) will be used to grade adverse events. At each study visit, participants will be questioned about adverse events they have experienced since the last study visit (using a standard list of known side effects of the two study drugs).
2. Secondary Outcome Measures
For the secondary and tertiary endpoints a participant will be classed as a treatment success if they have 3 or less significant blisters present on examination and have not had their treatment modified (changed or dose increased) on account of a poor response.
• Difference in the proportion of participants who are classed as a treatment success at 6 weeks.
• Difference in the proportion of participants in each treatment arm who are classed as treatment success at 6 weeks and are alive at 52 weeks. This measure will provide a good overall comparison of the two treatment arms . . . ” 50
Explanation
The trial outcomes are fundamental to study design and interpretation of results. For a given intervention, an outcome can generally reflect efficacy (beneficial effect) or harm (adverse effect). The outcomes of main interest are designated as primary outcomes, which usually appear in the objectives (Item 7) and sample size calculation (Item 14). The remaining outcomes constitute secondary or other outcomes.
For each outcome, the trial protocol should define four components: the specific measurement variable, which corresponds to the data collected directly from trial participants (e.g., Beck Depression Inventory score, all cause mortality); the participant-level analysis metric, which corresponds to the format of the outcome data that will be used from each trial participant for analysis (e.g., change from baseline, final value, time to event); the method of aggregation, which refers to the summary measure format for each study group (e.g., mean, proportion with score > 2); and the specific measurement time point of interest for analysis.163
It is also important to explain the rationale for the choice of trial outcomes. An ideal outcome is valid, reproducible, relevant to the target population (e.g., patients), and responsive to changes in the health condition being studied.67 The use of a continuous versus dichotomous method of aggregation can affect study power and estimates of treatment effect,164;165 and subjective outcomes are more prone to bias from inadequate blinding (ascertainment bias) and allocation concealment (selection bias) than objective outcomes.166;167 Although composite outcomes increase event rates and statistical power, their relevance and interpretation can be unclear if the individual component outcomes vary greatly in event rates, importance to patients, or amount of missing data.168-171
The number of primary outcomes should be as small as possible. Although up to 38% of trials define multiple primary outcomes,4;35;163 this practice can introduce problems with multiplicity, selective reporting, and interpretation when there are inconsistent results across outcomes. Problems also arise when trial protocols do not designate any primary outcomes, as seen in half (28/59) of protocols for a sample of trials published from 2002-2008,12 and in 25% of randomised trial protocols that received ethics approval in Denmark in 1994-95.4 Furthermore, major discrepancies in the primary outcomes designated in protocols/registries/regulatory submissions versus final trial publications are common; favour the reporting of statistically significant primary outcomes over non-significant ones; and are often not acknowledged in final publications.172-176 Such bias can only be identified and deterred if trial outcomes are clearly defined beforehand in the protocol and if protocol information is made public.177
Where possible, the development and adoption of a common set of key trial outcomes within a specialty can help to deter selective reporting of outcomes and to facilitate comparisons and pooling of results across trials in a meta-analysis.178-180 The COMET (Core Outcome Measures in Effectiveness Trials) Initiative aims to facilitate the development and application of such standardised sets of core outcomes for clinical trials of specific conditions (http://www.comet-initiative.org). Trial investigators are encouraged to ascertain whether there is a core outcome set relevant to their trial and, if so, to include those outcomes in their trial. Existence of a common set of outcomes does not preclude inclusion of additional relevant outcomes for a given trial.[ps2id id=’participant-timeline’ target=”/]
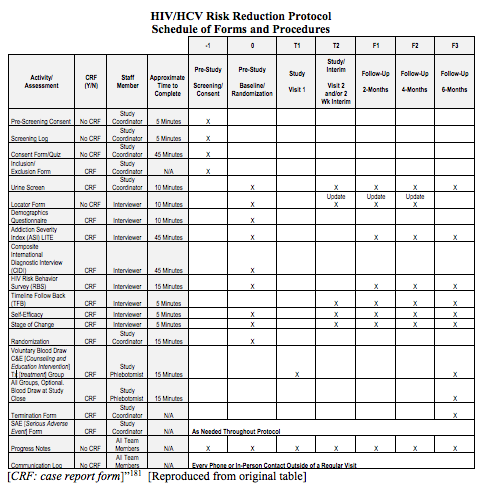
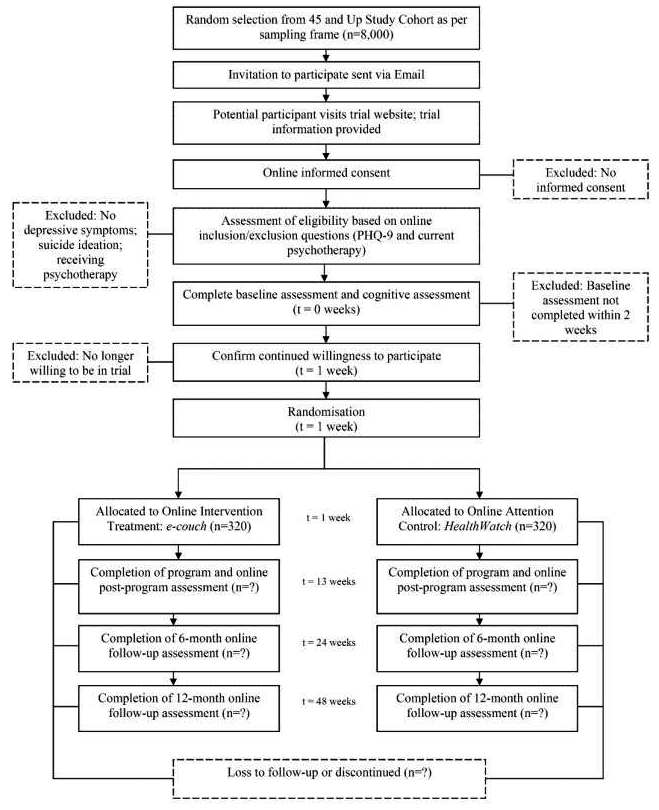
13. PARTICIPANT TIMELINE
Time schedule of enrolment, interventions (including any run-ins and washouts), assessments, and visits for participants. A schematic diagram is highly recommended (see Figure 1).
Example 1
“The main outcomes of interest are the drug and sex-related HIV [human immunodeficiency virus] and HCV [hepatitis C virus] risk behaviors . . . Clients will be assessed using the full battery of instruments from the Common Assessment Battery (CAB), along with the Self-Efficacy and Stages of Change questionnaires and a Urine Drug Screen after consenting . . . questionnaires will take place for all participants 14-30 days after randomization during which they will be given the Stages of Change and Self-Efficacy questionnaires, the Timeline Follow-Back, and a UA [urinalysis]. Follow-up interviews, using the full battery (CAB and questionnaires), will be collected at 2 months (56 days), 4 months (112 days) and 6 months (168 days) after the randomization date. A 14 day window, defined as 7 days before and 7 days after the due date, will be available to complete the 2 and 4 month follow-up interviews and a 28 day window, defined as 7 days before and 21 days after the due date, will be available to complete the 6 month follow up interview . . .
7.1.1 Common Assessment Battery (CAB)
A Demographic Questionnaire . . .
The Composite International Diagnostic Interview Version 2.1 . . .
The Addiction Severity Index-Lite (ASI-Lite) . . .
The Risk Behavior Survey (RBS) . . .
7.1.2 Additional Interviews/Questionnaires
To assess drug use, urinalysis for morphine, cocaine, amphetamine, and methamphetamine will be performed at the 2-Week Interim Visit, and the 2-, 4-, and 6-month Follow-up visits . . .
Stage of change for quitting drug use will be measured using a modification of the Motivation Scales . . .
Example 2
” 182 [Click to enlarge]
Explanation
A clear and concise timeline of the study visits, enrolment process, interventions, and assessments performed on participants can help to guide trial conduct and enable external review of participant burden and feasibility. These factors can also affect the decision of potential investigators and participants to join the trial (Item 15).91
A schematic diagram is highly recommended to efficiently present the overall schedule and time commitment for trial participants in each study group. Though various presentation formats exist, key information to convey includes the timing of each visit, starting from initial eligibility screening through to study close-out; time periods during which trial interventions will be administered; and the procedures and assessments performed at each visit (with reference to specific data collection forms, if relevant) (Figure 1).[ps2id id=’sample-size’ target=”/]
14. SAMPLE SIZE
Estimated number of participants needed to achieve study objectives and how it was determined, including clinical and statistical assumptions supporting any sample size calculations.
Example 1
“The sample size was calculated on the basis of the primary hypothesis. In the exploratory study [Reference X], those referred to PEPS [Psycho-education with problem solving] had a greater improvement in social functioning at 6 month follow-up equivalent to 1.05 points on the SFQ [Social Functioning Questionnaire]. However, a number of people received PEPS who were not included in the trial (e.g., the wait-list control) and, for this larger sample (N=93), the mean pre-post- treatment difference was 1.79 (pre-treatment mean=13.85, SD=4.21; post-treatment mean=12.06, SD=4.21). (Note: a lower SFQ score is more desirable). This difference of almost 2 points accords with other evidence that this is a clinically significant and important difference [Reference X]. A reduction of 2 points or more on the SFQ at 1 year follow-up in an RCT of cognitive behaviour therapy in health anxiety was associated with a halving of secondary care appointments (1.24.vs 0.65), a clinically significant reduction in the Hospital Anxiety and Depression Scale (HADS [Reference X]) Anxiety score of 2.5 (9.9 vs 7.45) and a reduction in health anxiety (the main outcome) of 5.6 points (17.8 vs 12.2) (11 is a normal population score and 18 is pathological) [Reference X]. These findings suggest that improvements in social functioning may accrue over 1 year, [sic] hence we expect to find a greater magnitude of response at the 72 week follow-up than we did in the exploratory trial. Therefore, we have powered this trial to be able to detect a difference in SFQ score of 2 points. SFQ standard deviations vary between treatment, control, and the wait-list samples, ranging from 3.78 to 4.53. We have based our sample size estimate on the most conservative (i.e., largest) SD [Standard deviation]. To detect a mean difference in SFQ score of 2 point (SD = 4.53) at 72 weeks with a two-sided significance level of 1% and power of 80% with equal allocation to two arms would require 120 patients in each arm of the trial. To allow for 30% drop out, 170 will be recruited per arm, i.e., 340 in total.” 183
Example 2
“Superficial and deep incisional surgical site infection rates for patients in the PDS II® [polydioxanone suture] group are estimated to occur at a rate of 0.12 [Reference X]. The trials by [Reference X] have shown a reduction of SSI [surgical site infections] of more than 50% (from 10.8% to 4.9% and from 9.2% to 4.3% respectively). Therefore, we estimate a rate of 0.06 for PDS Plus® [triclosan-coated continuous polydioxanone suture].
For a fixed sample size design, the sample size required to achieve a power of 1-β = 0.80 for the one-sided chi-square test at level α = 0.025 under these assumptions amounts to 2 × 356 = 712 (nQuery Advisor®, version 7.0). It can be expected that including covariates of prognostic importance in the logistic regression model as defined for the confirmatory analysis will increase the power as compared to the chi-square test. As the individual results for the primary endpoint are available within 30 days after surgery, the drop-out rate is expected to be small. Nevertheless, a potential dilution of the treatment effect due to drop-outs is taken into account (e.g. no photographs available, loss to follow up); it is assumed that this can be compensated by additional 5% of patients to be randomized, and therefore the total sample size required for a fixed sample size design amounts to n = 712 + 38 = 750 patients.
. . .
An adaptive interim analysis [Reference X] will be performed after availability of the results for the primary endpoint for a total of 375 randomized patients (i.e., 50% of the number of patients required in a fixed sample size design). The following type I error rates and decision boundaries for the interim and the final analysis are specified:
• overall one-sided type I error rate: 0.025
• boundary for the one-sided p-value of the first stage for accepting the null-hypothesis within the interim analysis: α0 = 0.5
• one-sided local type I error rate for testing the null-hypothesis within the interim analysis: α1 = 0.0102
• boundary for the product of the one-sided p-values of both stages for the rejection of the null-hypothesis in the final analysis: cα = 0.0038
If the trial will be continued with a second stage after the interim analysis (this is possible if for the one-sided p-value p1 of the interim analysis p1∈]0.0102,0.5[ [ie. 0.5≥p1≥0.0102] holds true), the results of the interim analysis can be taken into account for a recalculation of the required sample size. If the sample size recalculation leads to the conclusion that more than 1200 patients are required, the study is stopped, because the related treatment group difference is judged to be of minor clinical importance.
. . .
The actually achieved sample size is then not fixed but random, and a variety of scenarios can be considered. If the sample size is calculated under the same assumptions with respect to the SSI rates for the two groups, applying the same the overall significance level of α = 0.025 (one-sided) but employing additionally the defined stopping boundaries and recalculating the sample size for the second stage at a conditional power of 80% on the basis of the SSI rates observed in the interim analysis results in an average total sample size of n = 766 patients; the overall power of the study is then 90% (ADDPLAN®, version 5.0).” 100
Explanation
The planned number of trial participants is a key aspect of study design, budgeting, and feasibility that is usually determined using a formal sample size calculation. If the planned sample size is not derived statistically, then this should be explicitly stated along with a rationale for the intended sample size (e.g., exploratory nature of pilot studies; pragmatic considerations for trials in rare diseases).17;184
For trials that involve a formal sample size calculation, the guiding principle is that the planned sample size should be large enough to have a high probability (power) of detecting a true effect of a given magnitude, should it exist. Sample size calculations are generally based on one primary outcome; however, it may also be worthwhile to plan for adequate study power or report the power that will be available (given the proposed sample size) for other important outcomes or analyses because trials are often underpowered to detect harms or subgroup effects.185;186
Among randomised trial protocols that describe a sample size calculation, 4-40% do not state all components of the calculation.6;11 The protocol should generally include the following: the outcome (Item 12); the values assumed for the outcome in each study group (e.g., proportion with event, or mean and standard deviation) (Table 2); the statistical test (Item 20a); alpha (type 1 error) level; power; and the calculated sample size per group – both assuming no loss of data and, if relevant, after any inflation for anticipated missing data (Item 20c). Trial investigators are also encouraged to provide a rationale or reference for the outcome values assumed for each study group.187 The values of certain pre-specified variables tend to be inappropriately inflated (e.g., clinically important treatment effect size)188;189 or underestimated (e.g., standard deviation for continuous outcomes),190 leading to trials having less power in the end than what was originally calculated. Finally, when uncertainty of a sample size estimate is acknowledged, methods exist for sample size re-estimation.191 The intended use of such an adaptive design approach should be stated in the protocol.
Table 2. Outcome values to report in sample size calculation.[table nl=”~~” div style=”float:left” class=”table table-bordered” tablesorter=”0″ table delimiter=”|” ]
|
Element|Binary[attr width=”110″]|Continuous[attr width=”110″]|Time-to-event[attr width=”110″]
Assumed result for each study group| Proportion (%) with event| Mean and standard deviation| Proportion (%) with event at a given time point
Effect measure| Relative risk, odds ratio| Difference in means| Hazard ratio
[/table]
Note: Although the sample size calculation uses the anticipated outcome value for each group, it is also recommended to report the corresponding contrast between groups (estimated effect).
For designs and frameworks other than parallel group superiority trials, additional elements are required in the sample size calculation. For example, an estimate of the standard deviation of within-person changes from baseline should be included for crossover trials192; the intracluster correlation coefficient for cluster randomised trials193; and the equivalence or non-inferiority margin for equivalence or non-inferiority trials respectively.108;194 Such elements are often not described in final trial reports,110;195-198 and it is unclear how often they are specified in the protocol.
Complete description of sample size calculations in the protocol enables an assessment of whether the trial will be adequately powered to detect a clinically important difference.189;199-206 It also promotes transparency and discourages inappropriate post hoc revision that is intended to support a favourable interpretation of results or portray consistency between planned and achieved sample sizes[ps2id id=’recruitment’ target=”/].6;207
15. RECRUITMENT
Strategies for achieving adequate participant enrolment to reach target sample size.
Example
“Each center will screen subjects to achieve screening percentages of 50% women and 33% minority; screening will continue until the target population is achieved (12 subjects/site). We recognize that, because of exclusion by genotype and genotypic variation among diverse populations [Reference X], the enrolled cohort may not reflect the screened population. The enrollment period will extend over 12 months.
Recruitment Strategy:
Each clinical center involved in the ACRN [Asthma Clinical Research Network] was chosen based on documentation for patient availability, among other things. It is, however, worthy to note the specific plans of each center.
Harvard Clinical Center/Boston
. . . The Asthma Clinical Research Center at the Brigham & Women’s Hospital utilizes three primary resources for identifying and recruiting potential subjects as described below.
1. Research Patient Database
The Asthma Clinical Research Center at the Brigham and Women’s Hospital has a database of over 1,500 asthmatics . . .
2. Asthma Patient Lists . . .
3. Advertisements . . .
. . . the Madison ACRN site has utilized some additional approaches to target minority recruitment. We have utilized a marketing expert to coordinate and oversee our overall efforts in recruiting and retaining minorities . . . As a result of his efforts, we have advertised widely in newspapers and other publications that target ethnic minorities, established contacts with various ethnic community, university, church, and business groups, and conducted community-based asthma programs . . . For example, student groups such as AHANA (a pre-health careers organization focusing on minority concerns) will be contacted . . . In addition, we will utilize published examples of successful retention strategies such as frequent payment of subject honoraria as study landmarks are achieved and study participant group social events. Study visits will be carefully planned and scheduled to avoid exam-time and university calendar breaks . . .
The Harlem Hospital Center Emergency Department (ED) sees an average of eight adult patients per day for asthma. Through the REACH (Reducing Emergency Asthma Care in Harlem) project, we have . . . successfully recruited and interviewed 380 patients from the ED . . .
Responses to inquiries about participation in research studies are answered by a dedicated phone line that is manned during business hours and answered by voicemail at all other times. A research assistant responds to each inquiry immediately, using a screening instrument . . .
Patients are recruited for clinical trials at the Jefferson Center through two primary mechanisms: (1) local advertising; and (2) identification in the asthma patient registry (database). Local advertising takes advantage of the printed as well as the audio-visual media. Printed media include . . . All advertising in the printed and audio-visual media has prior approval of the Institutional Review Board.
The Jefferson patient registry (database) has been maintained since 1992 and currently contains 3,100 patients . . . It is estimated that 300-400 new asthmatic patients are seen each year, while a smaller number become inactive due to relocation, change of health care provider, etc. Once identified in the database, patients potentially eligible for a specific study are contacted by the nurse coordinator who explains the study and ascertains the patient’s interest. If interested, the patient is seen in the clinical research laboratories where more detailed evaluations are made . . .
Each subject will receive financial compensation within FDA [Food and Drug Administration] guidelines for participation in an amount determined by the local center. For subjects who drop out, payments will be pro-rated for the length of time they stayed in the study, but payment will not be made until the study would have been completed had the subject not dropped out.” 208
Explanation
The main goal of recruitment is to meet the target sample size (Item 14). However, recruitment difficulties are commonly encountered in clinical trials.209-213 For example, reviews of government funded trials in the US and UK found that two-thirds did not reach their recruitment targets.214;215 Low enrolment will reduce statistical power and can lead to early trial stoppage or to extensions with delayed results and greater costs.
Strategies to promote adequate enrolment are thus important to consider during trial planning. Recruitment strategies can vary depending on the trial topic, context, and site. Different recruitment methods can substantially affect the number and type of trial participants recruited128;209;216-220 and can incur different costs.221-223 Design issues such as the number and stringency of eligibility criteria will also directly affect the number of eligible trial participants.
Protocol descriptions of where participants will be recruited (e.g., primary care clinic, community), by whom (e.g., surgeon), when (e.g., time after diagnosis), and how (e.g., advertisements, review of health records) can be helpful for assessing the feasibility of achieving the target sample size and the applicability of the trial results in practice. Other relevant information to explicitly provide in the protocol includes expected recruitment rates, duration of the recruitment period, plans to monitor recruitment during the trial, and any financial or non-financial incentives provided to trial investigators or participants for enrolment (Item 4). If strategies differ by site in multi-centre trials, these should be detailed to the extent possible.